What is Catalyst QMS?
• Catalyst QMS is a Software Platform for Quality Management in Pharma, Med Devices, Biotech and related industries. It is a new
way of managing & controlling your Quality.
• It is a cloud based software platform which can acquire machine as well as user data.
• It is a validated system complying to the regulatory requirements of the life sciences industry.
• It Automates & standardizes all quality management processes and helps in tracking, improving & preventing defects
About Piton
Piton Systems Pvt Ltd is a Pune based software products company. Our founders have 20+ years’ experience working in companies like
Infosys and TCS and have done large enterprise ERP, QMS and other life sciences applications implementations
for global pharma clients.
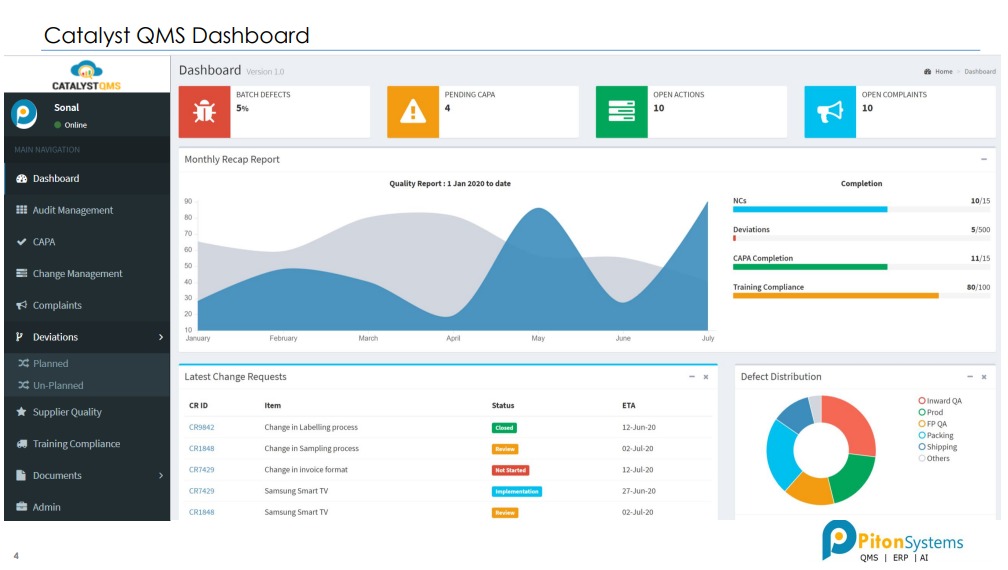
Benefits of Using Catalyst QMS
Tracking Quality (Workflows)
• Can acquire data from various sources like users and Machines avoiding human errors.
• Complete standardization of all Quality flows like Audits, CAPA, Change Mgmt, Deviations, etc.
• Gives complete control of Quality with 25-30% reduction in effort spent on Audits.
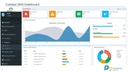
Improving Quality (Analytical Reports)
• Historical data of all defects, CAPA, audit findings, etc is available in easily searchable format.
• 20% improvement in quality due to use of readily available historical knowledge.
Preventing Poor Quality (AI Engine)
• As data builds in Catalyst, its AI engine kicks in. It uses data of all Quality events.
• It can then provide suggestions for events. e.g. which CAPA is most effective for a particular type of defect, or what are the most common causes of failure of a particular types of Batch, etc.
• This prevents the defects from occurring and further improves the quality
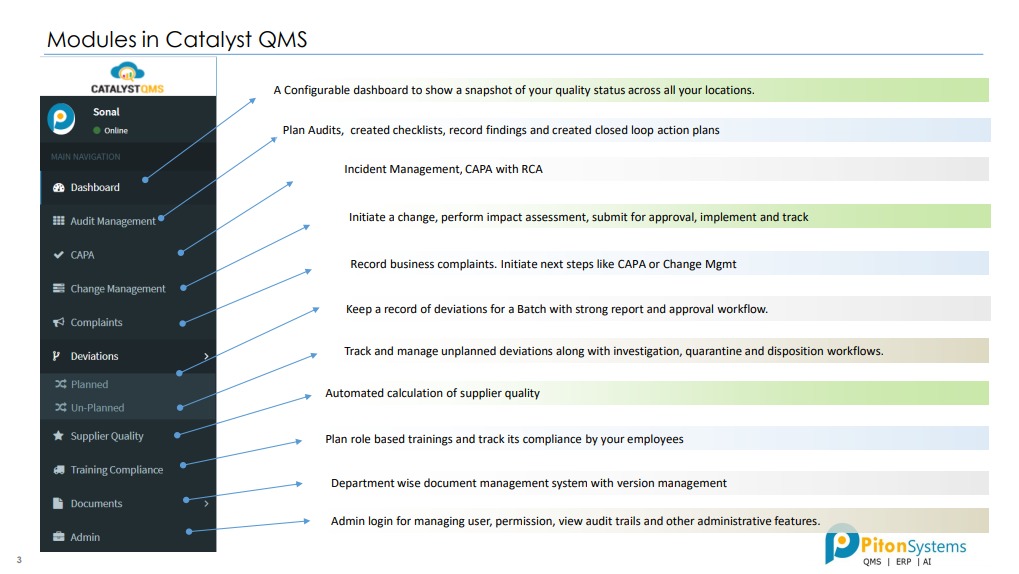
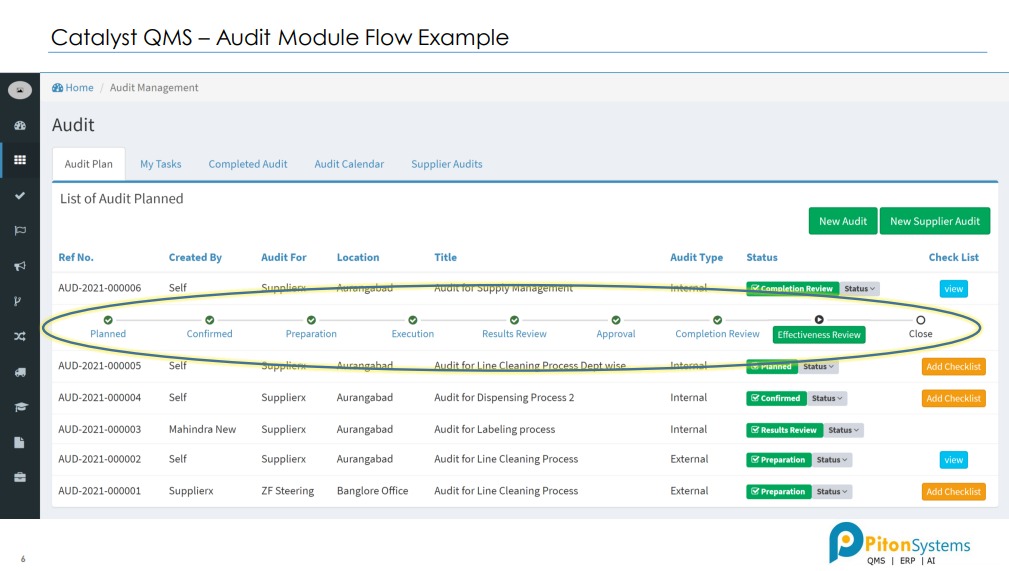
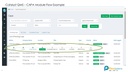
1. Audit Management
Plan Audits, created checklists, record findings and created closed loop action plans.
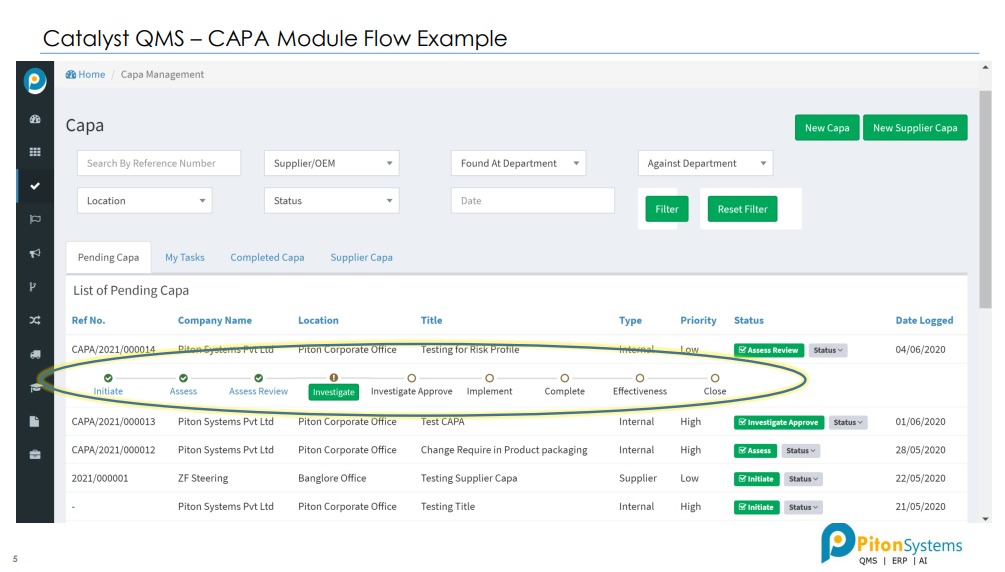
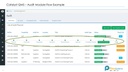
2. CAPA
Incident Management, CAPA with RCA.
3. Change Management
Initiate a change, perform impact assessment, submit for approval, implement and track.
4. Complaints
Record business complaints. Initiate next steps like CAPA or Change Mgmt.
5. Deviations
Track and manage unplanned deviations along with investigation, quarantine and disposition workflows.
6. Supplier Quality
Automated calculation of supplier quality.
7. Training Compliance
Plan role based trainings and track its compliance by your employees.
8. Documents
Department wise document management system with version management.
1- Audit Management System: Discover and resolve issues by building a continuously improving Quality Management System (QMS) by digitally transforming your audit management process – right from audit planning and preparation to audit report and closure
2- CAPA Module:
Drive continuous improvement (CI) of operations with a comprehensive yet easy-to-use system designed to effectively manage your corrective and preventive action (CAPA) processes.
3- Change management Module:
Navigate the complexity, coordination and execution of the change process lifecycle to drive continuous improvement.
4- Complaint Module:
Ensure customer delight through end-to-end management of complaints from in-take to assessing risks, regulatory impact, quality investigation, resolution, and continuous improvement
5- Deviation Module:
Reduce Cost of Quality (CoQ) from internal failure and prevent product recalls with an automated, data-driven process for identifying and managing nonconformance of both products and processes.
6- Supplier quality Module:
Streamline your supply chain output by integrating quality, safety, performance management and collaboration tools to increase supplier performance while reducing risk and cost.
7- Training Compliance Module:
Ensure your workforce is trained, skilled and certified to drive productivity and maintain compliance
8- Documents Module: Relevant Information within Reach – Always